Enantioselective construction of 3-substituted 3-amino-2-oxindoles containing an N,N-ketal skeleton via organocatalyzed aza-addition of isatin imines†
Abstract
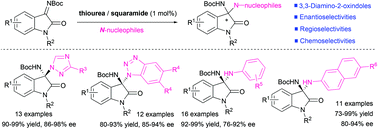
The first example of organocatalytic chemo-, regio- and enantioselective aza-Mannich reactions of triazoles and arylamines, respectively, with isatin-derived imines has been achieved in the presence of double hydrogen bonding organocatalysts, affording the valuable optically active 3-substituted 3-amino-2-oxindoles featuring N,N-ketal structural motifs in high yields. This strategy was featured by low catalyst loading, mild conditions, broad substrate scope, and high efficiency and selectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...