Highly enantioselective addition of sulfur-containing heterocycles to isatin-derived ketimines†
Abstract
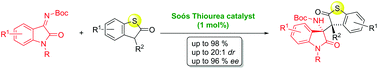
In this study, we report a highly stereoselective addition of sulfur-containing heterocyclic compounds to isatin-derived ketimines efficiently catalyzed by cinchonidine-derived bifunctional tertiary aminothiourea (1 mol%). This organocatalytic methodology furnishes a new type of optically active heterocyclic compound with two adjacent chiral quaternary carbon stereocenters in good yield (up to 98%), with excellent diastereoselectivity (up to 20 : 1 dr) and high enantioselectivity (up to 95% ee).
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...