Diethylaminophenyl-based Schiff base Cu(ii) and V(iv) complexes: experimental and theoretical studies and cytotoxicity assays†
Abstract
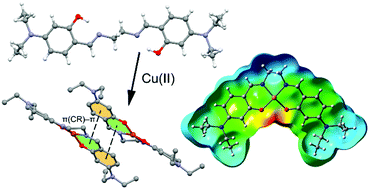
An N,N,O,O donor Schiff base blocking ligand [H2L = 6,6′-((1E,1′E)-(ethane-1,2-diylbis(azaneylylidene))bis(methaneylylidene))bis(3-(diethylamino)phenol), 1] has been used to synthesize mononuclear [CuL]·H2O (2) and [VO(L)] (3) complexes. The ligand and complexes have been characterized by elemental, spectral (FTIR and UV-vis) and thermogravimetric analysis. The molecular structures of 1 and 2 have been confirmed by single crystal X-ray diffraction studies. The crystal structure of the ligand (1) indicates that the molecule is sited on a crystallographic inversion centre and the planar conformation of the salicylideneimine moiety is favored by two intramolecular O–H⋯N1 hydrogen bonds forming S(6) ring motifs. The copper(II) center in complex 2 is coordinated in a square planar fashion. The presence of an extended π-system in the complex (two chelate rings and two phenyl rings) facilitates the formation of chelate ring (CR)⋯π non-covalent interactions. DFT calculations have been performed to explore the energetic features of unconventional CR⋯π and hydrogen bonding interactions that are observed and described in the solid state of 2. Moreover, we have studied how the energy of the CR⋯π interaction is influenced by the substituent of the phenyl ring. In addition, the cytotoxic effect of the compounds has been tested against MG-63 (human osteosarcoma), HT-29 (human colorectal) and MCF7 (breast) cancer cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.


 Please wait while we load your content...
Please wait while we load your content...