Effect of lithium and sodium ions on the size and morphology of ZnO nanoparticles synthesized by a glycerol–urea route†
Abstract
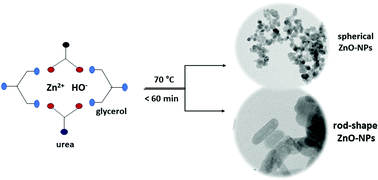
ZnO nanoparticles having spherical and rod shapes with high crystallinity and uniform average crystallite sizes were prepared by a new route named the glycerol–urea (GU) route. The syntheses were carried out using Zn(NO3)2·6H2O and NaOH in a 3 : 1 mol ratio of G : U solution under stirring at 70 °C. These reactions took less than 1 hour to completion with a high mass yield. NaCl and LiCl salts were added to attempt to modulate crystallites morphology and size. The effect of LiCl led to smaller spherical particles with an average size of 11(2) nm, whereas NaCl resulted in rod-shaped ZnO nanoparticles having an average length of 44(7) nm and an average diameter of 12(2) nm; whereas GU synthesis (no salt) led to 19(3) nm nanoparticles. Detailed X-ray powder diffraction, FT-IR and UV-Vis analyses confirmed the typical wurtzite structure of the particles. Electron microscopy images (SEM and TEM) showed the morphology and size of the ZnO nanoparticles. Photoluminescence revealed different behaviors related to defects and exciton emission.


 Please wait while we load your content...
Please wait while we load your content...