Metal-free, green and efficient oxidative α halogenation of enaminones by halo acid and DMSO†
Abstract
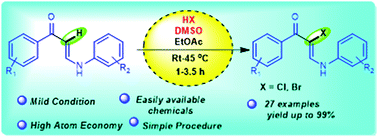
Metal free oxidative halogenation of N-aryl enaminones has been demonstrated using a DMSO–halo acid combination under mild reaction conditions. This strategy allows a facile halogenation of enaminones through α functionalization leading to a broad range of α-halo-N-aryl substituted enaminones in good to excellent yields in a short period of time. Additionally, the use of readily available and inexpensive HX and DMSO makes this system more (atom economical) practical. The present method is a straightforward approach and is also applied for the synthesis of chromenone derivatives in excellent yields.


 Please wait while we load your content...
Please wait while we load your content...