2-Methyl-substituted monotetrazoles in copper(ii) perchlorate complexes: manipulating coordination chemistry and derived energetic properties†‡
Abstract
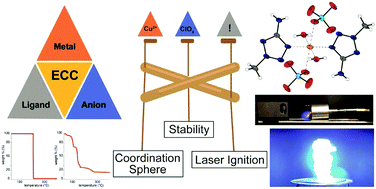
A proposed correlation between coordination chemistry and deduced energetic properties (thermal behaviour, and sensitivities towards mechanical and optical stimuli) of copper(II) complexes is investigated. Starting from a system comprising Cu(ClO4)2 and either of the ligands 2-methyl-5-aminotetrazole (1, 2-MAT) or 2-methyl-5H-tetrazole (2, 2-MTZ), typically altered parameters like the metal(II) centre, ligand, or counterion were predefined. Instead, solely slight changes in ligand concentration and the solvent system were implemented in order to provide an insight into structure–property relationships of energetic coordination compounds (ECC) of this type. As a result, five highly energetic complexes [Cu(H2O)2(2-MAT)4](ClO4)2·H2O (3), [Cu(H2O)2(2-MAT)4](ClO4)2 (4), [Cu(H2O)2(2-MAT)4](ClO4)2·2 2-MAT (5), [Cu(ClO4)2(H2O)2(2-MAT)2] (6), and [Cu(H2O)2(2-MTZ)4](ClO4)2 (7) were synthesized and, except for 5, elaborately characterized. Besides structural elucidation via X-ray diffraction, NIR-spectroscopy, differential thermal analysis (DTA), standard sensitivity measurements (impact, friction, and electrostatic discharge), UV/vis-spectroscopy, and optical initiation experiments were conducted to deduce a precise relationship between coordination chemistry and the consequential energetic characteristics of these complexes.


 Please wait while we load your content...
Please wait while we load your content...
