Silver sulfide nanoparticles in aqueous environments: formation, transformation and toxicity†
Abstract
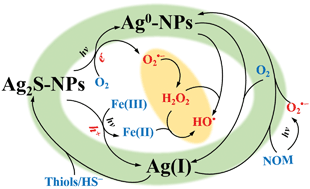
Sulfidation of silver nanoparticles (Ag-NPs) readily occurs in both urban sewage systems and sulfur-rich natural environments with formation of silver sulfide nanoparticles (Ag2S-NPs). It is essential to understand the transformation and fate of Ag2S-NPs in order to fully evaluate environmental impact of Ag-NPs. The review focuses on the formation and transformation of Ag2S-NPs from both thermodynamic and kinetic perspectives, particularly (i) the formation mechanism of Ag2S in various environmental scenarios, (ii) redox transformation of Ag2S caused by oxidation of S(−II) and (iii) effects of environmental matrices on the formation and transformation processes. In most cases, sulfidation of Ag-NPs causes a dramatic decrease in their toxicity due to the extremely low solubility of Ag2S, potentially restraining their short-term environmental impact. However, the transformation of Ag2S-NPs with potential release of Ag+ and in situ formation of Ag0 and Ag0/Ag2S-NPs hetero-nanostructures may possibly increase the toxicity. Mechanistically-based kinetic modeling has been proposed here to quantitatively describe the rate and extent of the transformation of Ag2S-NPs, with such models of value, at least, in validating proposed transformation mechanisms of Ag2S-NPs and, at best, in predicting their transformation behaviors under realistic environmental conditions.


 Please wait while we load your content...
Please wait while we load your content...