Slow magnetic relaxation in Dy2 and Dy4 complexes of a versatile, trifunctional polydentate N,O-ligand†
Abstract
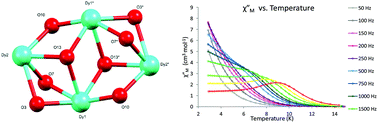
A tridentate ligand LH3 (C11H13N3O4) comprising o-vanillin, hydrazone and oxime donor groups has been employed to prepare a new class of di- and tetranuclear LnIII complexes. The reaction of LH3 with Ln(NO3)3·xH2O in the presence of a suitable base yields the dinuclear DyIII complex [Dy2(LH2)4(CH3OH)][NO3]2 (1) and the tetranuclear complexes [Dy4(LH)4(LH2)2(OH)2]·2H2O (2) and [Gd4(LH)4(LH2)2(OMe)2]·nH2O, (3). In these complexes, LH3 is either monodeprotonated (1) or a mixture of mono- and doubly-deprotonated ligands (2 and 3) binding lanthanide ions via Ndiazine, Ohydrazone, and Ovanillin donors, while the remaining vacant coordination sites are occupied by OMeOH (1), Ohydroxide (2) and methoxides (3). DC magnetic susceptibility studies on the isotropic tetranuclear GdIII complex (3) reveal weak antiferromagnetic exchange interactions between the LnIII ions. AC studies reveal that the dinuclear complex (1) exhibits field-induced slow relaxation of magnetization with Ueff = 43.4 K, whereas 2 is a single molecule magnet, exhibiting slow relaxation of magnetization under zero field below 18 K, which is modelled using a combination of Orbach (Ueff/kB = 26.7 K) and Raman relaxation processes.


 Please wait while we load your content...
Please wait while we load your content...