Influence of geometric isomerism on the binding of platinum anticancer agents with phospholipids†
Abstract
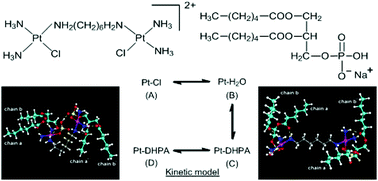
Reported herein is a detailed NMR and DFT study of the interaction of the 15N-labelled dinuclear platinum anticancer compound [{cis-PtCl(NH3)2}2{μ-H2N(CH2)6NH2}]2+ (15N-1, 1,1/c,c) with 1,2-dihexanoyl-sn-glycero-3-phosphate (DHPA), as a comparison with an earlier study of the interaction of the same water-soluble phospholipid fragment with the geometric trans isomer (1,1/t,t). The reaction of 15N-1 with the sodium salt of DHPA was studied at 298 K, pH ∼ 5.6, by [1H,15N] HSQC 2D NMR spectroscopy. The NMR data, supported by DFT models, provide evidence that the monofunctional DHPA adduct of 15N-1 exists in two conformational forms, with different orientation of the (CH2)6 linker; one has an interaction between the unbound {PtN3Cl} moiety and the coordinated DHPA molecule. Similarly, two bifunctional adduct conformers are identified, in which one has an interaction between the phosphate groups of the two bound DHPA molecules. When compared to the previously reported reactions of 1,1/t,t with DHPA, equilibrium conditions of the 1,1/c,c reaction are reached more slowly (120 h), similar to the reaction with phosphate. The rate constant for the first step of DHPA binding (kL) is slightly lower (1.6 fold) for the cis-compared to the trans-isomer, whereas the rate constant for the reverse reaction is 4-fold lower, resulting in a much greater proportion of DHPA bound species at equilibrium.
- This article is part of the themed collection: The central role of the d-block metals in the periodic table


 Please wait while we load your content...
Please wait while we load your content...