Rate coefficient of the reaction CH2OO + NO2 probed with a quantum-cascade laser near 11 μm†
Abstract
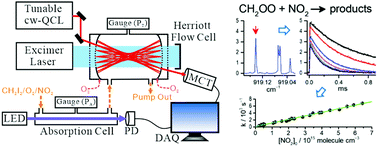
The reaction of the simplest Criegee intermediate CH2OO with NO2 is considered to be important in atmospheric chemistry because of its prospective contribution to the decay of CH2OO and the additional source of NO3, hence its effects on the NOx and HOx cycles. The reported rate coefficients of this reaction varied by a factor of 5. Employing a cw quantum-cascade laser with wavelength near 11 μm coupled with Herriott mirrors to probe CH2OO sensitively, we investigated in detail the reaction CH2OO + NO2 under total pressure 5.9–9.7 Torr at 298 K and reported a rate coefficient kNO2 = (1.0 ± 0.2) × 10−12 cm3 molecule−1 s−1, about one-seventh the value 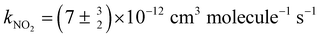 determined with direct measurements of CH2OO by Weltz et al. This smaller rate coefficient implies that the title reaction contributes to the atmospheric source of NO3 and the decay of CH2OO much less than previously conceived.
determined with direct measurements of CH2OO by Weltz et al. This smaller rate coefficient implies that the title reaction contributes to the atmospheric source of NO3 and the decay of CH2OO much less than previously conceived.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...