The key effects of polymorphism during PbII uptake by calcite and aragonite†
Abstract
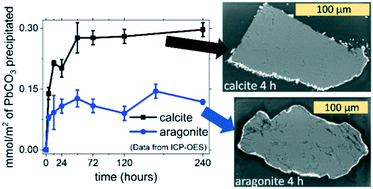
Lead can be extracted from contaminated water and fixed as a solid phase by a dissolution–precipitation reaction converting abundant Ca carbonates into sparingly soluble Pb carbonates. Herein, we investigate the mechanism of calcite (CAL) and aragonite (ARG) recrystallization in acidic Pb(NO3)2 solutions and demonstrate that the efficiency of this process depends strongly on the crystal structure of Ca carbonate used. The lower reactivity of aragonite is related to surface passivation by a continuous layer of cerussite, which is isostructural with ARG (both orthorhombic). The amount of CaCO3 converted into PbCO3 was determined for both CAL and ARG: after 10 days of interaction, 15 ± 2% of calcite and 6 ± 1% of aragonite were transformed into cerussite (maximum theoretical conversion yield of 50%: 2 mmol CaCO3(s) interacting with 1 mmol Pb(aq)). In situ atomic force microscopy (AFM) allowed the observation of the mechanism of calcite dissolution in the presence of Pb(NO3)2 solutions; we observed a stabilization of the steps along the [010] direction. Pb2+ ions were demonstrated to be responsible for the change in the morphology of the dissolving calcite surface; nitrate ions alone did not induce any similar distortion under the same conditions. Two different Pb-bearing phases were formed during the AFM experiments. The most abundant product (cerussite) was made of elongated crystals randomly distributed on the {104} surface of calcite. A minor amount of another Pb-bearing phase was observed; this hexagonal-shaped phase (hydrocerussite) was imaged while growing with a spiral mechanism along the c-axis.


 Please wait while we load your content...
Please wait while we load your content...