A two-step nucleation model based on diffuse interface theory (DIT) to explain the non-classical view of calcium carbonate polymorph formation
Abstract
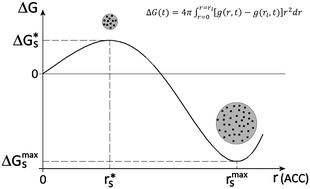
We developed a phenomenological two-step nucleation model based on diffuse interface theory (DIT) to explain the non-classical pathway of crystallization of calcium carbonate polymorphs (calcite, aragonite and vaterite): (i) initially, formation of an amorphous phase (amorphous calcium carbonate, ACC); (ii) later, formation of a crystalline phase within the amorphous one. Our model uses an arbitrary function describing the evolution of the Gibbs free energy of the system over time. We show that the amorphous cluster can nucleate, overcoming a low activation energy, and successively grow until a maximum size is reached (step I); the cluster cannot grow further, otherwise the Gibbs free energy of the system increases indefinitely. To reduce further the Gibbs free energy of the system, the crystalline phase nucleates within the amorphous cluster or at its interface (step II). We believe that the amorphous cluster can operate as a crystallization chamber, a nanometric environment suitable for crystal formation, where it is less expensive to form a crystalline phase than continuing to grow the amorphous cluster.


 Please wait while we load your content...
Please wait while we load your content...