Stoichiometric and electrocatalytic production of hydrogen peroxide driven by a water-soluble copper(ii) complex†
Abstract
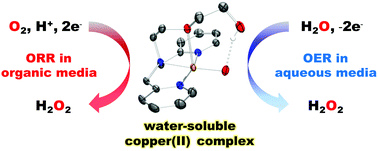
Herein, a water-soluble molecular copper complex was investigated as a catalyst for O2 reduction in both water and an organic solvent. Although the quasi-stoichiometric oxygen reduction reaction (ORR) for the formation of H2O2 was conducted in an organic solvent and revealed mechanistic insights into the ORR, the electrocatalytic production of H2O2 was achieved in an aqueous medium.


 Please wait while we load your content...
Please wait while we load your content...