Selective biocatalytic hydroxylation of unactivated methylene C–H bonds in cyclic alkyl substrates†
Abstract
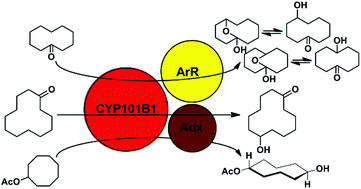
The cytochrome P450 monooxygenase CYP101B1 from Novosphingobium aromaticivorans selectively hydroxylated methylene C–H bonds in cycloalkyl rings. Cycloketones and cycloalkyl esters containing C6, C8, C10 and C12 rings were oxidised with high selectively on the opposite side of the ring to the carbonyl substituent. Cyclodecanone was oxidised to oxabicycloundecanol derivatives in equilibrium with the hydroxycyclodecanones.


 Please wait while we load your content...
Please wait while we load your content...