Bulk poly(N-isopropylacrylamide) (PNIPAAm) thermoresponsive cell culture platform: toward a new horizon in cell sheet engineering†
Abstract
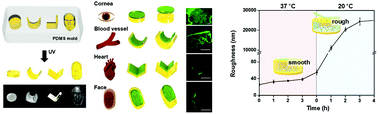
Although a conventional method of utilizing thermoresponsive grafted poly(N-isopropylacrylamide) (PNIPAAm) enables the harvest of a healthy confluent cell-to-cell junction preserved cell sheet while limiting the use of the trypsin enzyme, the absolute necessity in the delicate control of a sensitive nm-scale PNIPAAm chain length inevitably decelerates the advancement of cell sheet engineering. In this study, we demonstrate, for the first time, a thermoresponsive cell culture platform composed only of a ‘bulk’ form of a PNIPAAm hydrogel with the Young's modulus being increased up to the MPa scale. The surface roughness of the bulk PNIPAAm hydrogel initially modulated by the cross-linker concentration was altered from the nm- to μm-scale in response to a change in temperature above/below the low critical solution temperature (LCST) of 32 °C. The appropriate control of the surface roughness allowed the stable attachment (above the LCST) and easy detachment (below the LCST) of diverse cells and enabled the harvest of cell sheets composed of cell lines (C2C12 and NIH3T3) or even primary cells (human umbilical vein endothelial cells and keratinocytes). During their incubation at 37 °C, the cell lines were able to be attached on every surface of the prepared PNIPAAm cell culture platforms, whereas the primary cells were found to be only attached on a surface having a roughness below ∼30 nm. Furthermore, in the aspect of cell sheet detachment at the incubation temperature of 20 °C, the cell sheets composed of cell lines were fully detached from the surface of the platform having a roughness of ∼10 μm or higher, while the cell sheets composed of primary cells were entirely detached from the surface with a roughness of ∼19 μm or higher. Based on such behaviors of the diverse cells at a given surface roughness, this study further suggests a universal thermoresponsive cell culture platform which allows the harvest of all types of cells from cell lines to primary cells in a desired shape. Our suggested universal cell culture platform could play a powerful and versatile role in accelerating the advancement of cell sheet engineering.


 Please wait while we load your content...
Please wait while we load your content...