A new 3D organotypic model of ovarian cancer to help evaluate the antimetastatic activity of RAPTA-C conjugated micelles†
Abstract
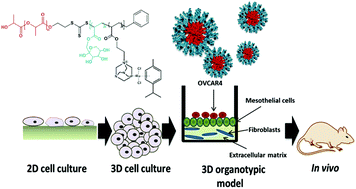
Introduction: Ovarian cancer is often diagnosed at a late stage, when disease has spread to extra-pelvic regions such as the omentum. There are limited treatment options available for women with extensive disease and tumours often relapse after current chemotherapy regimens. Therefore, novel drugs should be investigated for the treatment of ovarian cancer. A 3D organotypic model of ovarian cancer can provide a specific platform for the evaluation of nano-drugs. Using patient derived primary cells, the 3D model mimics the ovarian metastatic microenvironment allowing efficient and reproducible testing of many nanoparticles. Dichlororuthenium(II) (p-cymene) (1,3,5-triaza-7-phosphaadamantane) (RAPTA-C) conjugated fructose-micelles have been used as the promising nano-drug for the treatment of metastatic cancer. Therefore we aimed to investigate the anti-metastatic properties of RAPTA-C conjugated micelles in ovarian cancer metastasis. Methods: Ovarian cancer cell adhesion and invasion into a model of omentum were analyzed with and without RAPTA-C conjugated micelles in a range of conditions. Results: We observed that RAPTA-C showed low general toxicity to both primary healthy and cancer cell lines. RAPTA-C loaded micelles significantly enhance the internalization of ruthenium inside the cells compared to free drugs. RAPTA-C did not affect adhesion of OVCAR4 ovarian cancer cells; however, it significantly inhibited invasion of these cells within the omentum model, either in its free form or as cargos inside the micelles. However, when OVCAR4 were treated prior to implantation, invasion was not inhibited. Conclusion: A 3D organotypic model provides a clinically relevant and simple method to evaluate the efficiency of nano-drug treatment of ovarian cancer. The ability to inhibit metastasis of RAPTA-C delivered in fructose coated nanoparticles was investigated for the first time via this model. These results provide a good basis to continue the development of this nano-drug in vivo.


 Please wait while we load your content...
Please wait while we load your content...