Efficient red AIEgens based on tetraphenylethene: synthesis, structure, photoluminescence and electroluminescence†
Abstract
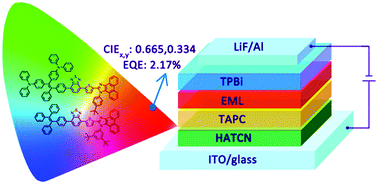
Red emitters are very important for colour displays and white lighting devices. However, efficient red emitters are relatively rare because they often suffer from the problem of aggregation-caused quenching. In this work, a series of robust red molecules consisting of tetraphenylethene, benzo-2,1,3-thiadiazole, phenanthro[9,10-d]imidazole and triphenylamine moieties are synthesized and fully characterized. The photophysical properties, transient fluorescence decay, thermal stability, and electrochemical behaviors and electronic structures are thoroughly investigated. The results show that these molecules have high thermal and electrochemical stabilities. They show aggregation-induced emission (AIE) properties and emit strong red fluorescence in the aggregated state, which can be well modulated by functional groups. Nondoped OLEDs are fabricated using these red molecules as light-emitting layers, offering red electroluminescence at 650 nm (CIEx,y = 0.665, 0.334) and a high luminance and an external quantum efficiency of up to 6277 cd m−2 and 2.17%, respectively. Moreover, a solution-processed red OLED with good performance is also achieved. This work not only presents efficient red emitters for nondoped OLEDs, but also provides useful structure–property relationship insights for further development of efficient red luminescent materials.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry C HOT Papers


 Please wait while we load your content...
Please wait while we load your content...