Intrinsic composition and electronic effects of multicomponent platinum nanocatalysts with high activity and selectivity for ethanol oxidation reaction†
Abstract
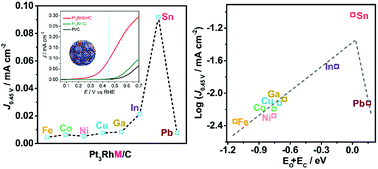
The sluggish kinetics of the ethanol oxidation reaction (EOR) challenges us to design and synthesize high-performance multicomponent nanocatalysts. Here, we report the intrinsic composition and electronic effects for achieving enhanced catalytic performance in the EOR by a combination of experiments and density functional theory (DFT) computations. Late 3d transition metals and main group (IIIA and IVA) metals were introduced to construct ternary Pt3RhM (M = Fe, Co, Ni, Cu; Ga, In, Sn, Pb) nanoalloys with similar geometric structures (nearly spherical) and crystallite sizes (ca. 8.5 nm) by a one-pot solvothermal method. The main group metals outperformed the transition metals in the enhancement of EOR activity and CO2 selectivity. In particular, Pt3RhSn/C exhibited 67- and 7-fold increases in specific activity and mass activity, respectively, at 0.45 V (vs. RHE) in the EOR in acidic conditions in comparison with a commercial Pt/C catalyst. The trends in catalytic activity were explained by DFT calculations, which established a volcano-shaped relationship between the EOR activity and the sum of the binding energies of oxygen and carbon (EO + EC). Among the above catalysts, the state-of-the-art Pt3RhSn/C catalyst performed best in terms of an optimum value of EO + EC with a moderate adsorption strength of stable intermediates. In addition, the CO2 selectivity was linearly correlated with the sum of the binding energies of oxygen and H2O (EO + EH2O), which closely matched the experimental results for typical catalysts. Therefore, the values of EO + EC and EO + EH2O served as descriptors of activity and selectivity, respectively, to rationalize and predict the chemical trends observed in experiments on the EOR catalyzed by nanoalloys. The concept that was revealed, namely, that the composition–performance relationship originates from the synergistic electronic effects of the nanoalloys, promises an alternative strategy for the development of novel solid catalysts for the EOR.


 Please wait while we load your content...
Please wait while we load your content...