Bifunctional electrocatalytic CoNi-doped manganese oxide produced from microdumbbell manganese carbonate towards oxygen reduction and oxygen evolution reactions†
Abstract
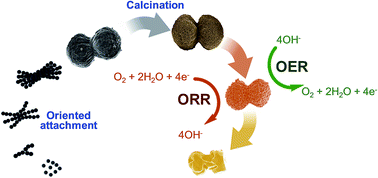
Herein, a new, simple, and stabilizer-free chemical synthetic protocol of microdumbbell manganese carbonate used as a precursor for producing nanoporous metal-doped manganese oxides is reported. This is the first time that a new growth mechanism of manganese carbonate via an oriented attachment mechanism of crystalline manganese carbonate nanospheres using its ferromagnetic property is revealed. The co-precipitation of divalent metal ions can be employed for the synthesis of metal-doped manganese carbonates. As-synthesized metal carbonates were employed as templates for producing porous metal-doped manganese oxides by calcination at 500–900 °C. The retention of template morphologies and metal oxide phases can be controlled by varying the calcination temperature. Doping of manganese oxide by Co2+ and Ni2+ to form a trimetal oxide can improve the catalytic performances for the ORR and OER under alkaline conditions. In addition, the trimetal oxide shows exceptional stabilities of 95.1% and 99.6% relative current retention for the ORR and OER at 40 000 s, respectively. The catalyst produced in this work may be practically used towards the ORR and OER in many systems such as metal–air batteries.


 Please wait while we load your content...
Please wait while we load your content...