Asymmetric synthesis of warfarin and its analogs catalyzed by C2-symmetric squaramide-based primary diamines†
Abstract
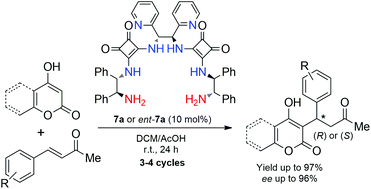
Novel C2-symmetric N,N′-bis(2-amino-1,2-diphenylethyl)squaramides with 1,2-di(pyridin-2-yl)ethane and 1,2-diphenylethane spacer groups were designed and applied as organocatalysts in asymmetric additions of 4-hydroxycoumarin and 4-hydroxy-6-methyl-2H-pyran-2-one to α,β-unsaturated ketones. Both enantiomers of the anticoagulant warfarin and its analogs were prepared in up to 96% yield and with 96% ee. Recyclability of the developed catalysts and synthetic utility of the prepared Michael adducts for asymmetric synthesis of potential chiral medications via acylation reactions were demonstrated.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...