Unveiling sequential late-stage methyltransferase reactions in the meleagrin/oxaline biosynthetic pathway†
Abstract
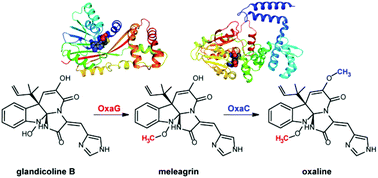
Antimicrobial and anti-proliferative meleagrin and oxaline are roquefortine C-derived alkaloids produced by fungi of the genus Penicillium. Tandem O-methylations complete the biosynthesis of oxaline from glandicoline B through meleagrin. Currently, little is known about the role of these methylation patterns in the bioactivity profile of meleagrin and oxaline. To establish the structural and mechanistic basis of methylation in these pathways, crystal structures were determined for two late-stage methyltransferases in the oxaline and meleagrin gene clusters from Penicillium oxalicum and Penicillium chrysogenum. The homologous enzymes OxaG and RoqN were shown to catalyze penultimate hydroxylamine O-methylation to generate meleagrin in vitro. Crystal structures of these enzymes in the presence of methyl donor S-adenosylmethionine revealed an open active site, which lacks an apparent base indicating that catalysis is driven by proximity effects. OxaC was shown to methylate meleagrin to form oxaline in vitro, the terminal pathway product. Crystal structures of OxaC in a pseudo-Michaelis complex containing sinefungin and meleagrin, and in a product complex containing S-adenosyl-homocysteine and oxaline, reveal key active site residues with His313 serving as a base that is activated by Glu369. These data provide structural insights into the enzymatic methylation of these alkaloids that include a rare hydroxylamine oxygen acceptor, and can be used to guide future efforts towards selective derivatization and structural diversification and establishing the role of methylation in bioactivity.
- This article is part of the themed collections: Chemical Biology in OBC and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...
