Biomimetic total syntheses of chromane meroterpenoids, guadials B and C, guapsidial A and psiguajadial D†
Abstract
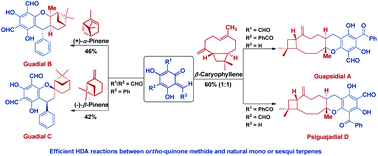
The first biomimetic total syntheses of chromane meroterpenoids, guadials B and C, guapsidial A and psiguajadial D have been completed. The key synthetic transformation involves an efficient and high yielding hetero-Diels–Alder reaction. The two structurally isomeric natural products, guadials B and C, were obtained from a common o-quinone methide in the separate reactions with α-pinene and β-pinene, respectively. The two regioisomeric natural products, guapsidial A and psiguajadial D, were achieved in a single chemical operation.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...