Base-mediated 1,6-conjugate addition of the Seyferth–Gilbert reagent to para-quinone methides†
Abstract
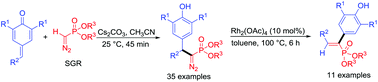
A 1,6-conjugate addition reaction of the Seyferth–Gilbert reagent (SGR) to p-quinone methides is reported. This base-mediated protocol allows rapid access to diarylmethylated diazomethylphosphonates. The reaction proceeds under mild basic conditions, making it a practical approach for the synthesis of diarylmethylated diazomethylphosphonates with a broad substrate scope. Interestingly, the treatment of the conjugate adduct with a catalytic amount of rhodium acetate resulted in the 1,2-aryl migration of the rhodium carbenoid intermediate to generate the corresponding 1,2-diaryl alkenylphosphonates in excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...