Interrogation of biosynthetic pathways of the cruciferous phytoalexins nasturlexins with isotopically labelled compounds†
Abstract
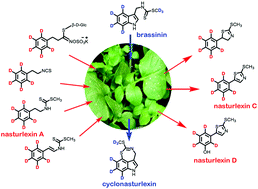
The discovery of the first non-indolyl cruciferous phytoalexins nasturlexins A and B together with cyclonasturlexin and brassinin, all chemical defenses of watercress plants (Nasturtium officinale R. Br.), revealed the co-occurrence of two parallel defense pathways, the tryptophan (Trp) pathway and the phenylalanine (Phe) pathway in crucifers. Similar to watercress, winter cress (Barbarea vulgaris R. Br.) and upland cress [B. verna (P. Mill.) Aschers] produce Phe derived phytoalexins, the nasturlexins C and D together with their counterpart sulfoxides. A detailed chemical understanding of the biosynthetic pathways of these phytoalexins facilitates their metabolic engineering. To this end, the biosynthetic pathways of cyclonasturlexin, nasturlexins A–D and corresponding sulfoxides in cress plants were investigated using isotopically labelled compounds. Except for the carbon atom of the thiomethyl groups of nasturlexins, the origin of all carbon atoms and nitrogen of nasturlexins was established to be homophenylalanine. A detailed map of the biosynthetic intermediates between phenylethyl isothiocyanates and nasturlexins A–D and sulfoxides in upland cress, winter cress and watercress is proposed. An application beyond these findings could lead to “designer crops” containing a wider range of chemical defenses that could make such crops more resistant to pests and diseases, a greatly advantageous trait.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...