Preparation of acetals from aldehydes and alcohols under basic conditions†
Abstract
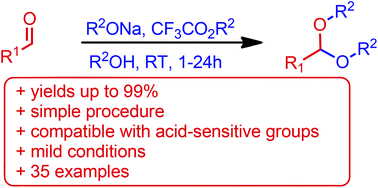
A new, simple protocol for the synthesis of acetals under basic conditions from non-enolizable aldehydes and alcohols has been reported. Such reactivity is facilitated by a sodium alkoxide along with a corresponding trifluoroacetate ester, utilizing formation of sodium trifluoroacetate as a driving force for acetal formation. The usefulness of this protocol is demonstrated by its orthogonality with various acid-sensitive protecting groups and by good compatibility with functional groups, delivering synthetically useful acetals complementarily to the synthesis under acidic conditions from aldehydes and alcohols.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...