Gold promoted arylative cyclization of alkynoic acids with arenediazonium salts†
Abstract
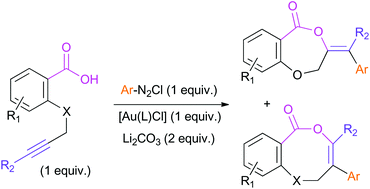
Alkynoic acids derived from salicylic acid and analogues undergo arylative cyclization with arenediazonium salts promoted by gold in the absence of external ligands. The reaction is thermally induced and proceeds even in the absence of light. A difference in regioselectivity has been found compared with that observed in the cycloisomerization process of the same type of compounds.


 Please wait while we load your content...
Please wait while we load your content...