Generic maps of optimality reveal two chemomechanical coupling regimes for motor proteins: from F1-ATPase and kinesin to myosin and cytoplasmic dynein
Abstract
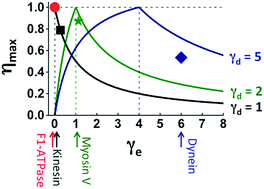
Many motor proteins achieve high efficiency for chemomechanical conversion, and single-molecule force-resisting experiments are a major tool to detect the chemomechanical coupling of efficient motors. Here, we introduce several quantitative relations that involve only parameters extracted from force-resisting experiments and offer new benchmarks beyond mere efficiency to judge the chemomechanical optimality or deficit of evolutionary remote motors on the same footing. The relations are verified by the experimental data from F1-ATPase, kinesin-1, myosin V and cytoplasmic dynein, which are representative members of four motor protein families. A double-fitting procedure yields the chemomechanical parameters that can be cross-checked for consistency. Using the extracted parameters, two generic maps of chemomechanical optimality are constructed on which motors across families can be quantitatively compared. The maps reveal two chemomechanical coupling regimes, one conducive to high efficiency and high directionality, and the other advantageous to force generation. Surprisingly, an F1 rotor and a kinesin-1 walker belong to the first regime despite their obvious evolutionary gap, while myosin V and cytoplasmic dynein follow the second regime. This analysis also predicts the symmetries of directional biases and heat productions for the motors, which impose constraints on their chemomechanical coupling and are open to future experimental tests. The verified relations, six in total, present a unified fitting framework to analyze force-resisting experiments. The generic maps of optimality, to which many more motors can be added in future, provide a rigorous method for a systematic cross-family comparison of motors to expose their evolutionary connections and mechanistic similarities.


 Please wait while we load your content...
Please wait while we load your content...