EvanPhos: a ligand for ppm level Pd-catalyzed Suzuki–Miyaura couplings in either organic solvent or water†
Abstract
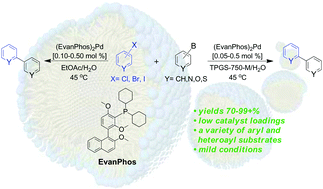
A new biaryl phosphine-containing platform can be constructed in only two steps. It complexes Pd(OAc)2 forming a precursor of a very active catalyst useful for Suzuki–Miyaura cross-couplings of functionalized substrates. By a combination of pre-activation, used together with the uncommon solvent EtOAc, the resulting catalyst system is effective at loadings in the ppm (0.1–0.5 mol%) range with highly functionalized reaction partners. Similar reactions run in water containing nanomicelles are as fast or faster. The derived Pd-complexed pre-catalyst possesses extended bench stability. The resulting technology represents an attractive green synthetic advance in highly valued Suzuki–Miyaura couplings.


 Please wait while we load your content...
Please wait while we load your content...
