Single pot selective hydrogenation of furfural to 2-methylfuran over carbon supported iridium catalysts†
Abstract
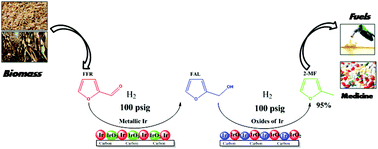
Various iridium supported carbon catalysts were prepared and screened for the direct hydrogenation of furfural (FFR) to 2-methyl furan (2-MF). Amongst these, 5% Ir/C showed excellent results with complete FFR conversion and highest selectivity of 95% to 2-MF at a very low H2 pressure of 100 psig. Metallic (Ir°) and oxide (IrO2) phases of Ir catalyzed the first step hydrogenation involving FFR to FAL and subsequent hydrogenation to 2-MF, respectively. This was confirmed by XPS analysis and some control experiments. At a low temperature of 140 °C, almost equal selectivities of FAL (42%) and 2-MF (43%) were observed, while the higher temperature (220 °C) favored selective hydrodeoxygenation. At optimized temperature, 2-MF was formed selectively while higher pressure and higher catalyst loading favored ring hydrogenation of furfural rather than side chain hydrogenation. With the combination of several control experimental results and detailed catalyst characterization, a plausible reaction pathway has been proposed for the selective formation of 2-MF. The selectivity to various other products in FFR hydrogenation can be manipulated by tailoring the reaction conditions over the same catalyst.


 Please wait while we load your content...
Please wait while we load your content...