Efficient photocatalytic water reduction by a cobalt(ii) tripodal iminopyridine complex†
Abstract
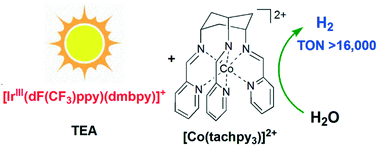
The visible light-driven catalytic water reduction by a cobalt(II) tripodal iminopyridine complex [Co(tachpy3)](ClO4)2 (1) (tachpy3 = cis,cis-1,3,5-tris(pyridine-2-carboxaldimino)-cyclohexane) has been investigated in aqueous acetonitrile. The catalysis is found to be homogeneous and an impressive H2 TON of 16 440 has been achieved, which is far better than that by a structurally related complex [Co(trenpy3)](ClO4)2 (2) (trenpy3 = tris-[4-(2-pyridyl)-3-azabut-3-enyl]amine) bearing identical donor groups. A comparison between the two complexes reveals that the catalytic properties are sensitive to the change in ligand rigidity and thus the coordination geometry, as reflected by their respective reaction potentials and H2 TONs. The catalysis of 1 proceeds via a penta-coordinate species formed by the protonation of the corresponding CoI state. The results in spectroscopic and electrochemical studies are supplemented by DFT computation.


 Please wait while we load your content...
Please wait while we load your content...