Effects of intramolecular hydrogen bonding and sterically forced non-coplanarity on organic donor/acceptor two-photon-absorbing molecules
Abstract
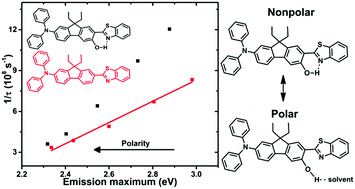
Two photon absorption (2PA) is of great interest across many disciplines and there has been a large effort to increase the two-photon cross section (σ2) via synthetic modification, especially by enhancing intramolecular charge-transfer (ICT). This work takes the previously studied (7-benzothiazol-2-yl-9,9-diethylfluoren-2-yl)diphenylamine (AF240), an asymmetric D–π–A chromophore, and intentionally appends a functional group (–OH, AF240-OH or –OCH3, AF240-OMe) to the 6-position of the fluorenyl π-bridge of the new chromophores. Electrochemical results in both dichloromethane and acetonitrile support stabilization of the highest occupied molecular orbital in the derivatives due to inductive electron donating effects of the hydroxy and methoxy groups. The lowest unoccupied molecular orbital is stabilized via intramolecular hydrogen bonding to the benzothiazole moiety in the case of AF240-OH. As previously observed for AF240, the steady-state emission spectra show significant solvatochromism as they broaden and red shift with increasing solvent polarity. The fluorescence lifetimes and quantum yields show that the non-radiative rate constant is increased for AF240-OH in all solvents, especially in nonpolar media. The results suggest there is forced intramolecular hydrogen bonding to the benzothiazole in nonpolar solvents because the solvent poorly solubilizes the hydroxy group. This increases the non-radiative decay rate constant (knr) via additional vibrational decay pathways. While not as dramatic, the increase in knr in polar solvents supports some deactivation via hydrogen bonding to the solvent. Steric effects are also observed in the methoxy derivative, which inhibits planarization of the benzothiazole with the fluorene, increasing the energy of the excited state. Ultrafast transient absorption spectroscopy in tetrahydrofuran solution supports stabilization of the excited state in a few ps as solvent and structural reorganizations occur. In the case of AF240-OH, no evidence of proton transfer is observed. The decrease in emission energies in the case of AF240-OH support increased ICT driven by higher degree of coplanarity and the quinoidal structure in the excited state. However, a moderate increase in the intrinsic 2PA cross-section is resulted. It is likely because of the two possible and competing solvent-stabilized ICT processes (PICT and TICT) in AF240-OH. Nevertheless, the strategic presence of a hydroxide group capable of intramolecular hydrogen bonding in AF240-OH provides a much broader 2PA sensitivity window than AF240.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
