The heat capacities and critical behaviors of binary ionic solutions†
Abstract
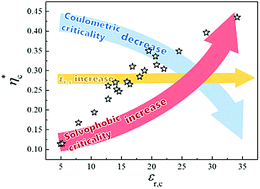
The heat capacities of nine binary room temperature ionic solutions {[C4mim][BF4] + 1,2-butandiol}, {[C8mim][BF4] + 1-pentanol}, {[C8mim][BF4] + 2-pentanol}, {[C8mim][BF4] + 1-hexanol}, {[C8mim][BF4] + 1-heptanol}, {[C8mim][PF6] + 1-propanol}, {[C8mim][PF6] + 1-butanol}, {[C8mim][PF6] + 2-butanol} and {[C8mim][PF6] + tert-butanol} are reported herein. The combination of the data obtained with the corresponding measured coexistence curves infers that the critical asymmetry parameter of the coexistence curves linearly varies with the molar volume ratio of the two components for each of the studied binary ionic solutions after the heat capacity contribution is considered. This indicates the importance of the heat capacity contribution to the critical asymmetry. For further analysis of the critical characteristics of the ionic solutions, a large amount of experimental data was collected and systematically discussed in detail regarding which critical character is important in the binary ionic solutions. A general increasing tendency for the RPM (restricted primitive model)-rescaled critical parameters with the relative permittivity εr,c of the solvent at the critical temperature of the corresponding system was found. This is attributed not only to the screening effect of the solvent medium, but also to solvophobic interactions, which both increase with εr,c. This study also demonstrates that the critical amplitudes increased, while the relative contribution of the heat capacity to the asymmetry of the coexistence curve decreased with an increase in εr,c.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...