Photoelectron spectroscopy and density functional theory studies of (FeS)mH− (m = 2–4) cluster anions: effects of the single hydrogen†
Abstract
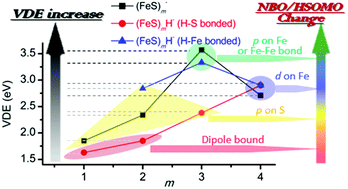
Single hydrogen containing iron hydrosulfide cluster anions (FeS)mH− (m = 2–4) are studied by photoelectron spectroscopy (PES) at 3.492 eV (355 nm) and 4.661 eV (266 nm) photon energies, and by Density Functional Theory (DFT) calculations. The structural properties, relative energies of different spin states and isomers, and the first calculated vertical detachment energies (VDEs) of different spin states for these (FeS)mH− (m = 2–4) cluster anions are investigated at various reasonable theory levels. Two types of structural isomers are found for these (FeS)mH− (m = 2–4) clusters: (1) the single hydrogen atom bonds to a sulfur site (SH-type); and (2) the single hydrogen atom bonds to an iron site (FeH-type). Experimental and theoretical results suggest such available different SH- and FeH-type structural isomers should be considered when evaluating the properties and behavior of these single hydrogen containing iron sulfide clusters in real chemical and biological systems. Compared to their related, respective pure iron sulfur (FeS)m− clusters, the first VDE trend of the diverse type (FeS)mH0,1− (m = 1–4) clusters can be understood through (1) the different electron distribution properties of their highest singly occupied molecular orbital employing natural bond orbital analysis (NBO/HSOMO), and (2) the partial charge distribution on the NBO/HSOMO localized sites of each cluster anion. Generally, the properties of the NBO/HSOMOs play the principal role with regard to the physical and chemical properties of all the anions. The change of cluster VDE from low to high is associated with the change in nature of their NBO/HSOMO from a dipole bound and valence electron mixed character, to a valence p orbital on S, to a valence d orbital on Fe, and to a valence p orbital on Fe or an Fe–Fe delocalized valence bonding orbital. For clusters having the same properties for NBO/HSOMOs, the partial charge distributions at the NBO/HSOMO localized sites additionally affect their VDEs: a more negative or less positive localized charge distribution is correlated with a lower first VDE. The single hydrogen in these (FeS)mH− (m = 2–4) cluster anions is suggested to affect their first VDEs through the different structure types (SH- or FeH-), the nature of the NBO/HSOMOs at the local site, and the value of partial charge number at the local site of the NBO/HSOMO.


 Please wait while we load your content...
Please wait while we load your content...