Approaching monocoordination at a silver(i) cation†
Abstract
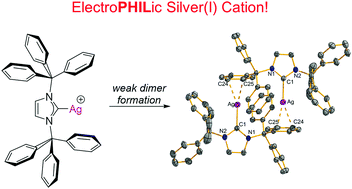
The recently reported bulky N-heterocyclic carbene ITr (ITr = [(HCNCPh3)2C:]) was found to stabilize low-coordinate Ag(I) environments. These electrophilic species were crystallographically identified as the weak solvates [(ITr)Ag(sol)]+ (sol = PhF, MesH or CH2Cl2) and as a solvent-free dimer [(ITr)Ag]22+. The highly electrophilic nature of the [(ITr)Ag]+ cation was further demonstrated by the calculation of a very high methyl ion affinity (MIA) and the synthesis of [(ITr)Ag(PCO)] which features substantial side-on electron donation from a P–C π bond to Ag.
- This article is part of the themed collection: Philip Power at 65: an icon of organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...