Cage carbon-substitute does matter for aggregation-induced emission features of o-carborane-functionalized anthracene triads†
Abstract
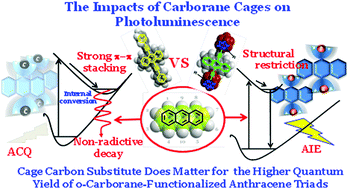
o-Carborane–anthracene triads were synthesized and characterized with anthracene and o-carborane acting as the chromophore and electron acceptor, respectively. Two functional groups were linked by an acetylene unit. The influence of the alkyl substituents in the cage carbon on the aggregation-caused quenching (ACQ) characteristics of the anthracene core was investigated. The results showed that the fluorescence quantum yield of the triads could be modulated from 7.0 to 42.9% in the solid state as the cage C substituents changed from H to methyl to ethyl. Mechanistic investigation indicated that the alkyl substitutes in the cage carbon could effectively suppress the Ccage–Ccage vibration, o-carborane unit rotation and intermolecular π–π stacking interactions. In addition, the 2-Me-o-carboranyl moiety could obviously twist the anthracene backbone, which was in favor of the twisted intramolecular charge transfer (TICT) state emission. These were responsible for the high fluorescence quantum yield in solid state.


 Please wait while we load your content...
Please wait while we load your content...