Designing highly efficient dual-metal single-atom electrocatalysts for the oxygen reduction reaction inspired by biological enzyme systems†
Abstract
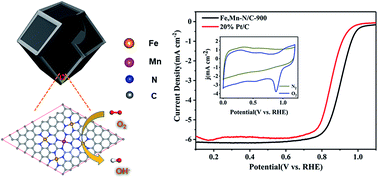
Biological heme–copper oxidases (HCOs) play a critical role in the four-electron, four-proton reduction of O2 to H2O in biosystems. HCOs exhibit high enzymatic activity due to their natural structure with heme–non-heme metal active sites, and the non-heme metal plays a role in conferring and fine-tuning the O2 reduction activity of the HCOs. Inspired by this binuclear active enzyme, herein, we designed an efficient electrocatalyst (Fe, Mn–N/C) for the oxygen reduction reaction, which contains two types of metal–Nx active site incorporated within the graphene framework of porous carbon. The catalyst displayed remarkable ORR performance with a half-potential of 0.904 V and kinetic current density of 33.33 mA cm−2, which is 4.9 times that of 20% Pt/C (6.76 mA cm−2). When the Fe, Mn–N/C catalyst was applied as an air electrode in a Zn–air battery, it exhibited a superior performance compared to commercial Pt/C. Its discharge curve showed that the change in output voltage was negligible at 20 mA cm−2 for 23 000 seconds (6.4 h). First principles calculations revealed that Fe, Mn–N/C needs less energy for the protonation of O* to OH* in ORR procedures compared with Fe–N/C. This catalyst, with its bimetal reactive center mimicking a metal enzyme, will pave a new way to design efficient electrocatalysts for the ORR in fuel cells.


 Please wait while we load your content...
Please wait while we load your content...