Heteroatomic TexS1−x molecule/C nanocomposites as stable cathode materials in carbonate-based electrolytes for lithium–chalcogen batteries†
Abstract
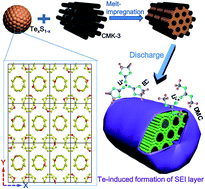
A stable cathode material in a conventional carbonate-based electrolyte for high-energy lithium–chalcogen batteries was successfully fabricated by homogeneously confining heteroatomic TexS1−x molecules into ordered mesoporous carbon CMK-3 via a facile melt-impregnation route. The Te–S bonds in the heteroatomic TexS1−x molecules endow them with higher intrinsic electrical conductivity and electrochemical reaction activity with Li than the homoatomic S8 molecules. Moreover, Te-containing polychalcogenide intermediates could induce the formation of solid electrolyte interphase (SEI) layers on the TexS1−x/CMK-3 surfaces in the carbonate-based electrolyte, which efficiently prevent polychalcogenides from the shuttle effect and side reactions with the carbonate solvent. With further assistance of mesopore confinement of CMK-3, the TexS1−x/CMK-3 composites can be reversibly charged and discharged in the carbonate-based electrolyte with long cycling stability and high rate capability. Therefore, the Te0.1S0.9/CMK-3 composite with an optimal Te/S mole ratio of 1/9 maintains high reversible capacities of 845 mA h g−1 after 100 cycles at 250 mA g−1 and 485 mA h g−1 after 500 cycles at 1 A g−1. These encouraging results suggest that the heteroatomic TexS1−x molecule/C composite could be a promising cathode material for long cycle life and high power density lithium batteries.


 Please wait while we load your content...
Please wait while we load your content...