An overall water-splitting polyoxometalate catalyst for the electromicrobial conversion of CO2 in neutral water†
Abstract
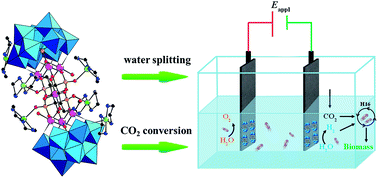
Electromicrobial conversion of carbon dioxide is a promising alternative to the direct transfer of CO2 into biomass by using bacteria that consume hydrogen and CO2 for living. The efficiency of such a hybrid system strongly depends on the electrocatalyst used for hydrogen production. Here we evidence that a mixed noble metal free Co/Cu-containing polyoxometalate/Carbon Cloth (Cu6Co7/CC) hybrid possesses excellent activity as a water splitting pre-electrocatalyst at neutral pH, with Hydrogen Evolution Reaction (HER) and Oxygen Evolution Reaction (OER) overpotentials of 390 mV and 500 mV at 10 mA cm−2 and Tafel slopes of 96 mV dec−1 and 147 mV dec−1, respectively. The nature of the catalysts involved for both the oxidation and reduction processes has been investigated. The long-term activity of the electrodes is also demonstrated. A negligible amount of H2O2 is produced during the electrolysis process. CO2 fixation with wild-type R. eutropha and the biocompatible Cu6Co7/CC precatalyst as both anode and cathode materials has been tested. The hydrogen produced on the cathode is consumed by the bacteria for living and growth with more than 50% of input electrical energy transferred into biomass. The solar-to-biomass efficiency ηSCE can reach 9.9 ± 0.5% in a 6-day experiment, almost 10 times higher than those of natural plants, showing the high performance of this Cu6Co7/CC/R. eutropha system.


 Please wait while we load your content...
Please wait while we load your content...