Highly functional energetic complexes: stability tuning through coordination diversity of isomeric propyl-linked ditetrazoles†
Abstract
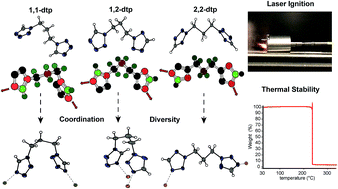
Currently used primary explosives suffer not only from various drawbacks like insidious sensitivities toward mechanical stimuli and electrostatic discharge, but also from environmental concerns largely attributed to toxic lead compounds. These issues can directly be related to higher risk during processing and handling of these sensitive materials. In this research, 12 new lead-free energetic coordination compounds (ECCs) based on three isomeric propyl-linked ditetrazoles as ligands with moderate sensitivities are described, which can be initiated reliably and safely by irradiation with near infrared light (NIR). Excellent thermal stabilities for all complexes up to an outstanding decomposition temperature of 297 °C for compound 7 could be achieved through the formation of stable polymeric networks. The optical and energetic properties of these complexes can easily be customized by variation of various building blocks like different transition metals (Mn2+, Fe2+, Ni2+, Co2+, Cu2+, Zn2+, and Ag+) and anions (perchlorate, styphnate, cyanodinitromethanide and dinitramide) and for the first time by the use of three different isomeric ditetrazole ligands. 1,3-Di(tetrazol-1-yl)propane (1,1-dtp), 1-(tetrazol-1-yl)-3-(tetrazol-2-yl)propane (1,2-dtp) and 1,3-di(tetrazol-2-yl)propane (2,2-dtp) were prepared in a convenient and straightforward one-step alkylation reaction of 1,5H-tetrazole. The obtained compounds were extensively characterized by e.g. XRD, IR, EA, UV/Vis and DTA. In addition, the sensitivities toward external stimuli (impact, friction and electrostatic discharge) were determined according to Bundesamt für Materialforschung und -prüfung (BAM) standard methods. Iron(II) 5 and copper(II) perchlorate complexes 8, 11 and 12 show promising characteristics and could be potential candidates for possible applications in the future.


 Please wait while we load your content...
Please wait while we load your content...
