A highly active and durable iron/cobalt alloy catalyst encapsulated in N-doped graphitic carbon nanotubes for oxygen reduction reaction by a nanofibrous dicyandiamide template†
Abstract
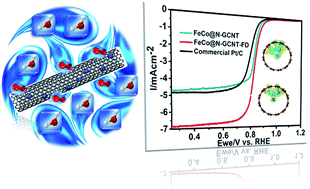
Exploration of competitive electrocatalysts to replace Pt-based catalysts for oxygen reduction reaction (ORR) in fuel cells is one of the most promising strategies to confront the energy and environmental crises. Herein, we highlighted an FeCo alloy catalyst encapsulated in N-doped graphitic carbon nanotubes (FeCo@N-GCNT-FD) as a highly efficient non-precious electrocatalyst for the ORR. The FeCo@N-GCNT-FD catalyst exhibits a positive onset (0.96 V vs. RHE) and half-wave potential (0.88 V vs. RHE) as well as 5.6 times the specific activity of commercial Pt/C at 0.70 V in alkaline media. The excellent catalytic behavior of FeCo@N-GCNT-FD is attributed to the structural properties, including a large surface area, and the synergistic effect of FeCo alloy and N-GCNT, which guarantee a large number of accessible catalytic sites and rapid mass-transfer kinetics. Theoretical calculations further confirm that the strong synergistic and electronic effects, especially the FeCo-NG sites, provide a favorable local coordination environment and electronic structure and a lower oxygen absorbance energy. The improvement of ORR activity and durability of the catalyst by the synergistic and electronic effects between the metal and carbon provides a versatile approach for tuning the catalytic performance of non-noble electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...