Mesoporous Ca–Mn–O as an efficient scavenger toward organic pollutants and heavy metals: ion exchange provoking ultrafast Fenton-like reaction based on the synergy of alkaline earth/transition metals†
Abstract
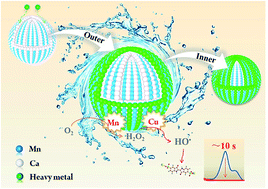
Intricate Fenton-like catalysts composed of mixed transition metal oxides (TMOs) frequently outperform simple TMOs in scavenging organic contaminants in wastewater. Nonetheless, their activity and/or reusability may sharply decline when abundant heavy metal ions in wastewater adsorb on the catalytic surface and passivate it. This study shows that mesoporous Ca–Mn mixed oxides prepared from a facile combination of Mn and an alkaline earth metal (i.e., Ca) (denoted as CaMnx) can readily overcome this problem. CaMnx without any additive (e.g., H2O2) was able to degrade organic contaminants (e.g., indigo carmine as a model contaminant) fast within 10 seconds, outperforming many other reported heterogeneous Fenton catalysts. Besides organics, the coexisting heavy metal ions (e.g., Cu2+ and Pb2+ as indicators) were also rapidly extracted. The outstanding performance was attributed to the synergy of Ca and Mn. CaO in CaMnx efficiently extracted metal ions via an ion exchange process, and MnOx generated abundant H2O2 and degraded the organics through an uncommon Fenton-like reaction. Therein, Cu species extracted by CaO catalyzed the decomposition of H2O2 into HO˙ radicals, speeding up the degradation reaction of the organics by 81 times. Benefiting from the gradual exposure of fresh Mn-sites protected by unreacted CaO, CaMnx without regeneration treatment maintained high efficiency in scavenging organics and heavy metal ions in cycling tests. CaMnx also showed great promise in treating complex water samples containing various heavy metal ions (e.g., Pb2+, Cu2+, Cd2+, Ag+, Zn2+ and Cr3+) and organic pollutants. The findings of this work enrich the rational design of related functional nanomaterials.
- This article is part of the themed collection: 2018 Journal of Materials Chemistry A HOT Papers


 Please wait while we load your content...
Please wait while we load your content...