Photoelectrochemical reduction of CO2 to HCOOH on silicon photocathodes with reduced SnO2 porous nanowire catalysts†
Abstract
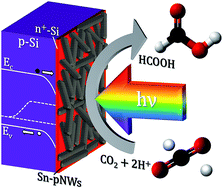
Electrochemical reduction of CO2 to liquid products offers a route for the energy-dense storage of intermittent renewable electricity while simultaneously helping to mitigate greenhouse gas emissions. In this work, high-quality Si photocathodes decorated with an earth-abundant Sn porous nanowire catalyst utilized the energy from visible light absorption to provide a photovoltage-assisted conversion of CO2 to liquid HCOOH. The Sn porous nanowire catalysts were selected for their high density of grain boundaries which was previously shown to enhance activity for formic acid formation. A faradaic efficiency of ∼60% with a partial current density of 10 mA cm−2 for HCOOH was achieved at −0.4 V vs. RHE under illumination, which reflected a positive potential shift of ∼400 mV compared to the dark electrocatalytic behavior. The photo-assisted electrolysis efficiency for formic acid was calculated to be 11.0%. The results represent a promising photocathode for a narrow bandgap subcell for a tandem photoelectrode system for unbiased light-driven CO2 electroreduction.


 Please wait while we load your content...
Please wait while we load your content...