Thermo-electrochemical cells empowered by selective inclusion of redox-active ions by polysaccharides†
Abstract
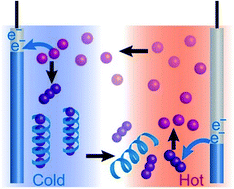
Thermo-electrochemical cells (TECs) are a class of thermoelectric materials that offer high thermoelectric voltage (Seebeck coefficient) with potentially lower costs compared to the conventional thermoelectric materials. To maximize the potential of TECs, we show that the Seebeck coefficient of TECs with a redox pair of I−/I3− is enhanced by introducing polymer–ion interactions. Starch and polyvinylpyrrolidone (PVP) are employed as polymeric hosts for I3− ions. The effective concentration of free I3− ions in the cold cell decreases due to their selective inclusion in host polymers, resulting in an increase of the [I−]/[I3−] ratio. Meanwhile in the higher temperature cell, the inclusion of I3− ions by host polymers is less effective and the [I−]/[I3−] ratio is mostly determined by the intrinsic equilibrium without polymers. Consequently, the two electrode cells differing in temperature show a considerable difference in the concentration of I3− ions, which causes a significant increase of the Seebeck coefficient up to 1.5 mV K−1. The performance of polymer TECs can be tuned depending on the polymer–I3− interactions, and starch showed notable performance as compared to PVP, with increased output power by a factor of two.


 Please wait while we load your content...
Please wait while we load your content...