One-pot one-base conditions for two shots: organocatalyzed tandem isomerization–olefination of allylic alcohols†
Abstract
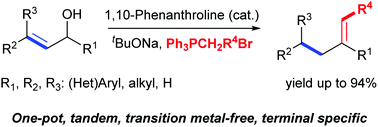
A one-pot isomerization–olefination of allylic alcohols using 1,10-phenanthroline as an organocatalyst in the presence of a base has been developed. Under one-pot one-base conditions, allylic isomerization and olefination were accomplished simultaneously. Either terminal or internal alkenes could be prepared in generally good to high yields from allylic alcohols without the involvement of transition metals or oxidants. This avoids the heavy metal and toxic residues in the resulting products and allows the applications of this method in pharmaceutical synthesis.


 Please wait while we load your content...
Please wait while we load your content...