Transition-metal-free and halogen-free controlled synthesis of poly(3-alkylthienylene vinylene) via the Horner–Wadsworth–Emmons condensation reaction†
Abstract
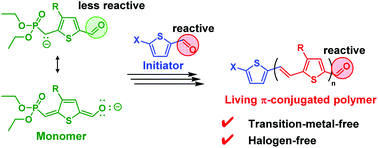
Transition metal catalysts and halogens are necessary for the controlled synthesis of π-conjugated polymers; the contamination by transition-metal-based and halogen-based byproducts generally adversely affects the performance of organic devices. In this study, we demonstrate a transition-metal-free and halogen-free controlled synthesis of poly(3-alkylthienylene vinylene) (P3ATV) via Horner–Wadsworth–Emmons polycondensation. The electrophilicity of the formyl group in the AB-type monomer, (3-(2-ethylhexyl)-5-formyl-thiophene-2-ylmethyl)phosphonic acid diethyl ester, could be deactivated by resonance effect using the appropriate base (NaHMDS/15-crown-5-ether). The initiator, 5-nitro-2-thiophenecarboxaldehyde, which contains the active formyl group, readily initiated the polymerization to afford well-defined P3ATV in a chain-growth manner. The molecular weights of the polymers were controlled (1800–10 200) by the feeding monomer/initiator ratio while maintaining narrow molecular weight distributions (1.20–1.28). Furthermore, the chain extension by post-polymerization could proceed effectively, highlighting the quasi-living nature of the proposed method.


 Please wait while we load your content...
Please wait while we load your content...