Cardanol-based polymer latex by radical aqueous miniemulsion polymerization†
Abstract
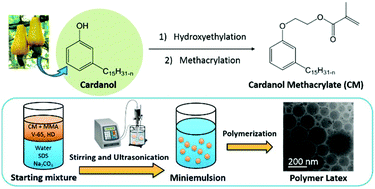
The facile one-pot, two-step synthesis of a new bio-sourced monomer derived from cardanol and its radical aqueous miniemulsion polymerization are presented in this work. As an abundant and renewable resource that does not compete with the food supply, cardanol is a compound of great interest for the replacement of petroleum-based materials due to its unique structure and properties. The new cardanol methacrylate (CM) was synthesized through hydroxyethylation of the phenolic hydroxyl group followed by methacrylation. The kinetics of the radical homopolymerization of CM and its copolymerization with methyl methacrylate (MMA) were investigated in toluene at 70 °C. The aqueous miniemulsion homo- and copolymerizations of CM and MMA were carried out at 20 wt% solids content, at 70 °C using 2,2′-azobis(2,4-dimethylvaleronitrile) as a radical initiator, sodium dodecyl sulfate as a surfactant, and hexadecane as a hydrophobe. The latexes were colloidally stable with monomodal particle size distributions and mean particle diameters ranging from 100 to 245 nm. The physical and chemical properties of the resulting polymer films were studied by thermogravimetric analyses and rheology.


 Please wait while we load your content...
Please wait while we load your content...