A naked-eye fluorescent sensor for copper(ii) ions based on a naphthalene conjugate Bodipy dye†
Abstract
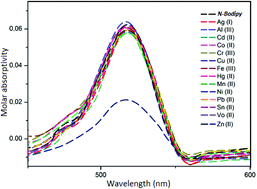
A novel naphthalene-Bodipy dye (N-Bodipy) was designed, prepared and characterized. N-Bodipy showed a selective and sensitive recognition toward Cu(II) ions as a fluorescent antenna group in acetonitrile/water over other metal cations. The complexation between Cu(II) ions and N-Bodipy gave a specific color change as well as caused fluorescence quenching under long-wavelength light (365 nm). The remarkable quenching effect in fluorescence intensity centered at 538 nm was only observed in the presence of copper(II) ions. Moreover, the orange color of N-Bodipy solution turned pale-yellow depending on the complexation effect in daylight. The complex stoichiometry was determined using a Job's plot and it was found to be 2 : 1 (ligand/metal). The binding constant was calculated with the Benesi–Hildebrand equation to be 1.39 × 1010 M−1 and the detection limit was 1.28 μM (LOD = 3α/slope, α is the standard deviation) for Cu(II). The data proved that the binding between N-Bodipy and Cu(II) is chemically reversible.

 Please wait while we load your content...
Please wait while we load your content...