1,2,4,5-Benzenetetracarboxylic acid: a versatile hydrogen bonding template for controlling the regioselective topochemical synthesis of head-to-tail photodimers from stilbazole derivatives†
Abstract
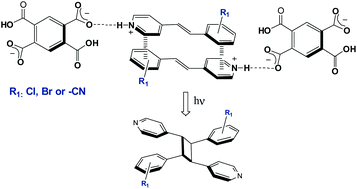
The crystal engineering of hydrogen bonded organic assemblies based on 1,2,4,5-benzenetetracarboxylic acid (H4bta) and stilbazole derivatives (1–10) is exploited to provide regio-controlled [2 + 2] photocycloadditions in the solid state. Single crystal X-ray diffraction analyses have revealed that all the arrays are built-up from the self-assembly of the (H2bta)2− dianion with two stilbazolium cations via O–H⋯O− and N+–H⋯O− charge-assisted H-bonding synthons: (4-Hstilbazolium+)2(H2bta2−). The dianion displays an interesting diversity of H-bonding motifs. Such structural flexibility allowed us to obtain four structure-types defined by the preferential formation of intramolecular or intermolecular hydrogen bonds between carboxylate-carboxylic groups. In these ionic assemblies two predominant structural H-bonding patterns were observed. The first pattern is characterised by the formation of intramolecular H-bonds in the dianion, leading to discrete assemblies based on ternary arrays. The second hydrogen pattern consists of 2-D hydrogen networks built-up from the self-assembly of anions via intermolecular H-bonds that are linked to the cations. Two additional examples, in which the dianion is self-assembled in two types of ribbons, were also observed. Another supramolecular feature predominant in all these arrays is the stacking of the cations in a head-to-tail fashion, which is controlled via cation–π interactions. These arrays are photoactive in the solid state upon UV-irradiation leading to the regioselective synthesis of rctt-cyclobutane head-to-tail-isomers in high to quantitative yield. In this work, the template tolerance either to steric or electronic effects by changing the number or positions of the supramolecular interactions exerted by distinctive functional groups was also explored. In addition, assemblies bearing 2-chloro (7 and 8) and 3-chloro-4-stilbazole (1 and 9) crystallize in two different crystalline forms, leading to novel examples of supramolecular isomers with similar solid state reactivity.

 Please wait while we load your content...
Please wait while we load your content...