Divergent synthesis of new α-glucosidase inhibitors obtained through a vinyl Grignard-mediated carbocyclisation†
Abstract
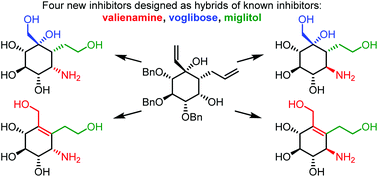
Four new α-glucosidase inhibitors have been synthesised through 5–8 synthetic steps from a common synthetic intermediate obtained through a recently developed carbocyclisation. The compounds were designed as hybrids of the known glucosidase inhibitors valienamine, voglibose and miglitol. All four compounds showed activity against rat intestinal sucrase with the most potent inhibitor acting at low micromolar concentration. The newly synthesised compounds were not as potent as miglitol against sucrase but showed greater selectivity towards the tested glycosidases. The most potent inhibitors were docked into a homology model built for this study of rat intestinal sucrase explaining the difference in potency between two diastereoisomers with varying orientation of a secondary amine.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...