Computational study of the mechanism of amide bond formation via CS2-releasing 1,3-acyl transfer†
Abstract
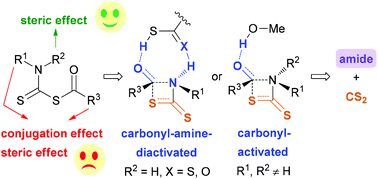
Reactions of thiocarboxylic acids and dithiocarbamate-terminal amines provide a linker-traceless method for amide bond formation under mild conditions, whereas the reaction mechanism is not clear. A systematic study was performed herein with density functional theory (DFT) calculations to elucidate the detailed mechanism, the substitution effect on the proposed CS2-releasing 1,3-acyl transfer and the differences between CS2- and CO2-releasing 1,3-acyl transfer. Relevant results indicate that this type of reaction proceeds via the nucleophilic addition of an in situ generated dithiocarbamic acid on thiocarboxylic acid, H2S elimination, rate-determining 1,3-acyl transfer and CS2 release. For the generation of secondary amides via the 1,3-acyl transfer, a thiocarboxylic acid- or dithiocarbamic acid-assisted pathway, in which both the carbonyl group and amide nitrogen are activated, is the most favored. For the generation of tertiary amides, MeOH-assisted carbonyl-activation is the most favorable pathway. N,N-Dialkyl substitution of the mixed anhydride intermediate promotes the 1,3-acyl transfer by the steric effect. In contrast, N-phenyl substitution and using thiobenzoic acid as a substrate slow down 1,3-acyl transfer by both the conjugation effect and steric effect. Furthermore, CS2-releasing 1,3-acyl transfer was found to be favored over CO2-releasing 1,3-acyl transfer in the aspects of both kinetics and thermodynamics mainly because the S–COR bond is weaker than the O–COR bond.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...